Pharmaceutical Emulsions: What are Emulsions, Types of Emulsion with examples, what are the Prons and Cons of using Emulsion Dosage form. In today’s series of my ‘Pharmaceutical Dosage forms’ I will explain in details all you need to know about Emulsion and method of preparing them.
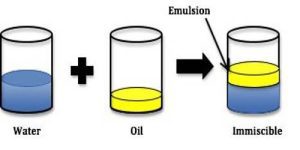
Emulsions are liquid preparation which are useful in oral route administration of drug and enema, they can be either be an oil in water emulsion (O/W), water in Oil Emulsion (W/O) or multiple emulsions (water in oil in water (w/o/w) or oil in water in oil (o/w/o)).
Read Also: Polytechnics That Offers Pharmacy In Nigeria
Overview of Emulsions
Important terms to familiarize yourself with
Internal Phase/Discontinuous phase:
This is the liquid droplets that is finely subdivided and uniformly dispersed in the external phase, it is also called the “Dispersed phase”.
External Phase/Continuous phase:
The liquid in which the droplets or globules are dispersed in. It is also called the dispersed medium.
Emulsifying Agent:
Are third party agents whose function is to stabilise the droplets of the internal phase.
With an understanding of these terms we can now begin properly what emulsions are, Types and method of preparation… ENJOY!
What is an Emulsion
Emulsions can be seen as a heterogeneous dispersed system which is made up of two immiscible liquid (water and oil), in which one of the liquid phase (internal phase) is broken down and uniformly distributed in the other (External Phase), this system is thermodynamically unstable and as such would require a third component called the ‘Emulsifying agent’.
This Emulsifying agent then help to stabilize the thermodynamically unstable system, I will talk more on Emulsifying agent on another blog post, Stay tuned.
Other names for Emulsifying agents are Emulgent or Emulsifier
Read Also: Universities That Offers PharmD Program In Nigeria
Types Of Emulsion
Depending on the external and internal phase, an emulsion can be classified into three (3)
- Oil In Water Emulsion (This is shorten as O/W)
- Water in Oil Emulsion (W/O)
- Multiple Emulsion (O/W/O or W/O/W)
Oil In Water O/W Emulsion:
In this type of Emulsified system, Oil is dispersed in water, therefore, you can say, Oil is the internal or Discontinuous phase, while water is the External or continuous phase.
Most pharmaceutically prepared emulsions intended to be administered orally must be in the Oil in water type of emulsion.
Water In Oil W/O Emulsion:
Here, the aqueous phase is dispersed as globules or droplet in the oily phase.
Multiple Phase Emulsion:
A multiple emulsion is formed when minute oil globules are dispersed in the water globules of a way to water in Oil Emulsion, this might then be described as an Oil-in-water-in-oil emulsion (O/W/O).
Theories of emulsion
Based on the type of emulsifying Agent used and certain Conditions of the internal and expernal phase such as pH, Physicochemical nature and proportions, four (4) theories of emulsion have been established. They are…
- Surface tension theory
- Interfacial film theory
- Oriented wedge theory by Bancroft harbens
- Formation of electrical double layer of charges (Stern layer)
The Surface Tension Theory
When two immiscible liquid are shaken together in the same system (container) and allowed to stand for a while, separation occurs with a distinct layer between the two immiscible liquids.
The use of surfactants results in reduction in the interfacial tension of the two immiscible liquids, reducing the repellant force between the liquids and diminishing each liquids attraction for its own molecules.
Interfacial film theory
This theory proposes that the emulsifier forms an interface between the oil and water, surrounding the droplets of the u ternak phase as a thin layer of film adsorbed on the surface of the droplets.
The more rigid or tougher the film is, the greater the stability of the emulsion.
Oriented-wedge theory
This theory was by Bancroft Harben, according to him, surfactants form macromolecular layers around droplets of the internal phase of the emulsion.
It is based in the assumption that emulsifying agents orient themselves about and within a liquid relative to their solubility in that particular liquid.
Formation of Electrical double layer of charges
This theory proposes that, in systems where there is free ionic charges in the preparations, a layer of similar ionic charges are deposited on the surface of the droplets and this layer of charges is closely followed by another layer of charges that have opposite charges to the initial layer.
How Emulsion Are aplied pharmaceutically
Liquid emulsion are used parentally, externally or orally. Emulsions intended for Oral administration are invariably of the oil-in-water type.
- Are used in the formation of depot preparations for IM administration
- Are used in x-ray diagnostic work
- May be used with other other nutrients to feed patient intravenously.
- Intestinal adsorption may be enhanced when globules are homogenised to below one micrometer in diameter.
This will be all for now, I understand you may have a question or two to ask, feel free to drop them using the comment box below!
Ensure to share this with friends on Facebook, Whatsapp, or any other social media network you can connect them with…
Related Searches a. emulgent pharmacy b. emulgent vs emulsifying agent c. emulgent cream d. emulgent example

![Universities That Offers PharmD Program In Nigeria [UPDATED] Pharm. D Program](https://drugsavant.com/wp-content/uploads/2021/06/images_1.jpeg)